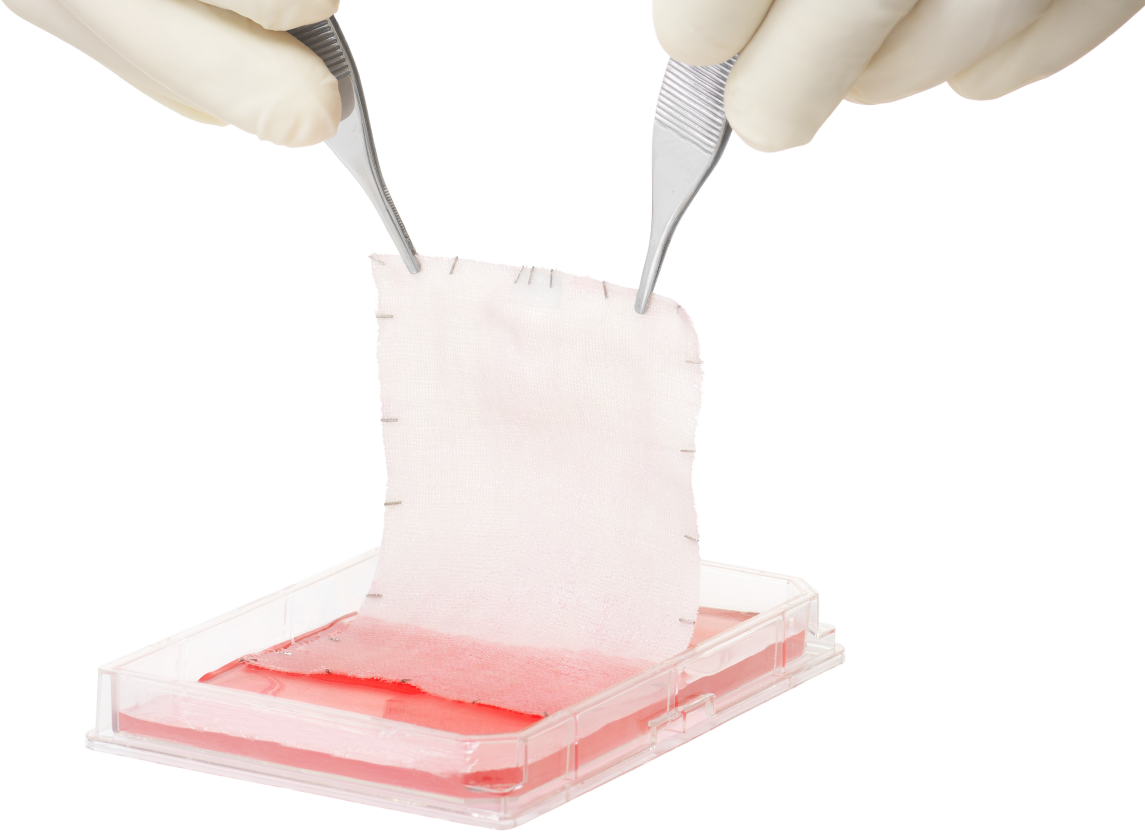
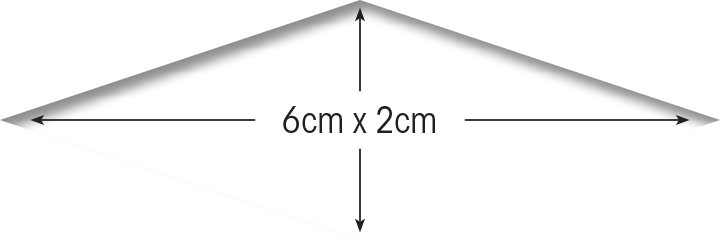
Epicel is permanent skin covering for burns ≥30% TBSA
Epicel Patient Survival
In the Epicel Clinical Experience databases, 954 adult and pediatric patients with a mean TBSA of 67.5% showed:
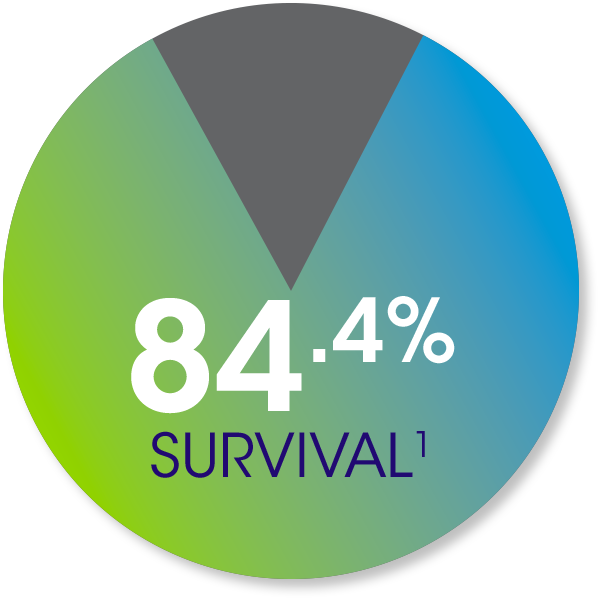
The overall survival rate was 84.4% (804/954) at hospital discharge.
The survival rate for pediatric patients was 88.9% and for adults the survival rate was 82.0%.1
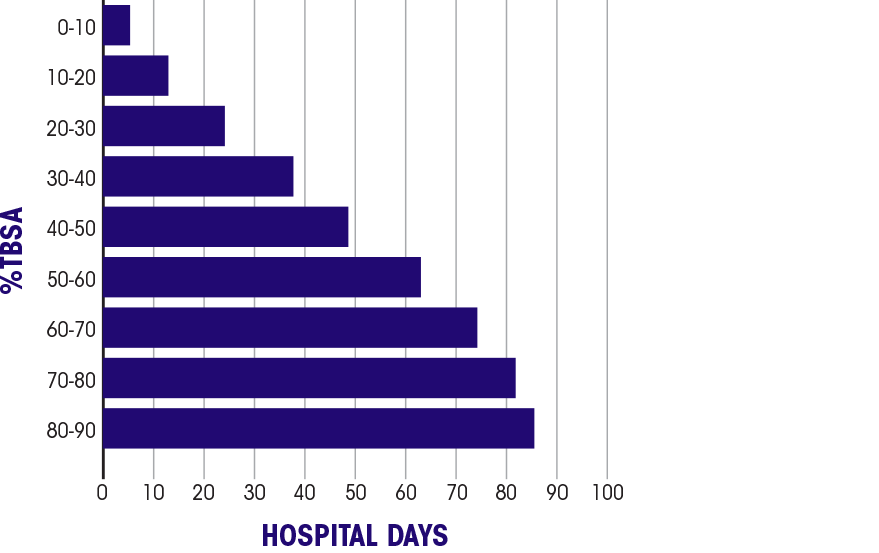
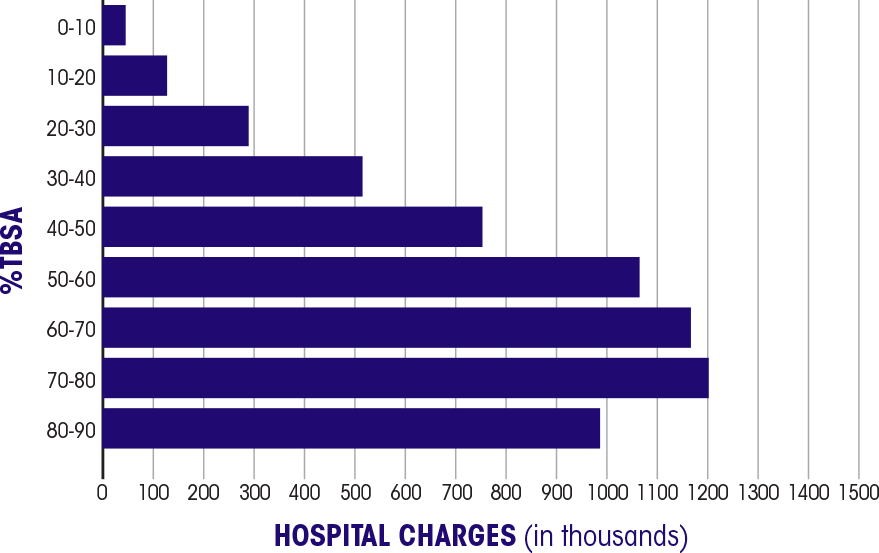
Burn patient survival In the 2016 National Burn Repository, 8,870 burn patients with a TBSA of 30%-90% showed: 68% survival2