Use Epicel on top of autograft for superior take rate and durability
Treatment
Apply Epicel over autograft meshed at 4:1– 6:1 ratio for best results in durability, take rate and cosmesis7

Epicel is indicated for adult and pediatric patients who have deep dermal or full thickness burns comprising a total body surface area greater than or equal to 30%
Use Epicel on top of autograft for superior take rate and durability
Apply Epicel over autograft meshed at 4:1– 6:1 ratio for best results in durability, take rate and cosmesis7
Use Epicel to achieve naturalized signalizing using the patient's own skin
If no autograft is available, apply Epicel directly over well-engrafted and vascularized allodermis/other dermal substitutes
Use Epicel to maximize donor skin for functional areas
Use Epicel to conserve autograft for use on critical areas meshed at a lower ratio (face, hands and joints)
Epicel can support the use of wide-mesh autograft from 4:1 up to 6:1
Healing occurs as the meshed autograft spaces fill in with cultured epidermis
A CEA/wide mesh autograft combination may increase the initial durability of Epicel5
Donor sites may also be covered with Epicel to accelerate reharvest potential8

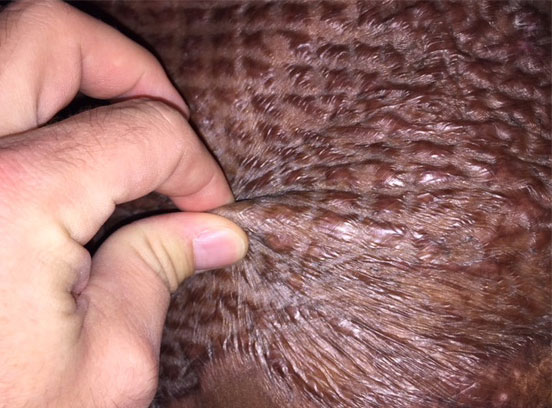
*Individual results may vary.
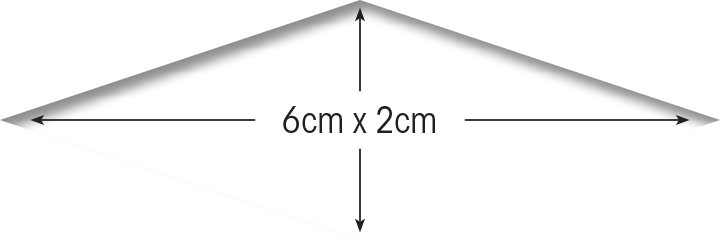
The autologous keratinocytes used to prepare Epicel (cultured epidermal autografts) are derived from two small, full-thickness biopsies of skin taken from an unaffected area on the burn patient
By using a patient's own multi-layer skin, once engrafted
Epicel can provide the patient with functional tissue4
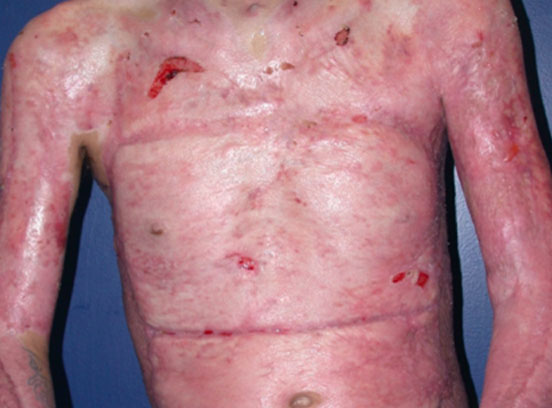
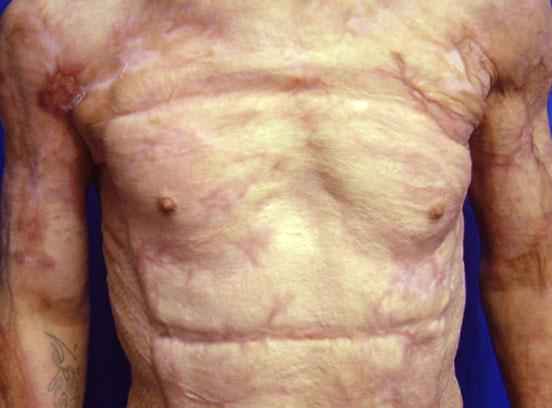
*Individual results may vary.
Click Here to Download the Epicel Treatment & Care Guidelines
Epicel is widely reimbursed and included on a majority of national insurance plans.
Click here to learn more about Epicel reimbursement