Large Burns
≥30% TBSA
Cookie Notice By continuing to use this website, you consent to our use of cookies and the other terms of our Privacy Policy.
For more information, please visit our Privacy Policy.
Epicel (cultured epidermal autografts) is authorized for use in adult and pediatric patients who have deep dermal or full thickness burns comprising a total body surface area greater than or equal to 30%. It may be used in conjunction with split-thickness autografts, or alone in patients for whom split-thickness autografts may not be an option due to the severity and extent of their burns. The effectiveness of the device for this use has not been demonstrated.
Epicel is contraindicated in patients with a history of anaphylaxis following exposure to vancomycin, amikacin, and amphotericin, as trace quantities of these anti-infective agents may remain in the Epicel autograft. Epicel should not be used in patients with known sensitivities to materials of bovine or murine origin. It is contraindicated for use on clinically infected wounds.
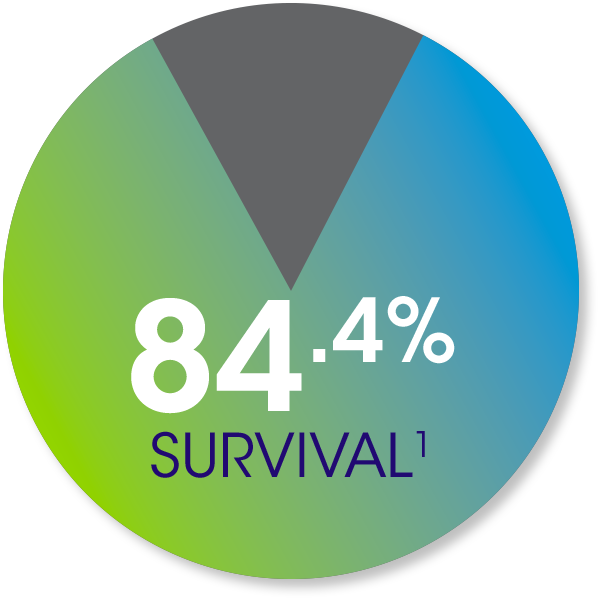
In the Epicel Clinical
Experience databases,
954 adult and pediatric
patients with a mean
TBSA of 67.5% showed:
In the 2016 National Burn Repository, 8,870
burn patients with a TBSA of 30%–90% showed: 68% survival2
In the Epicel Clinical Experience databases, 453 patients showed a median take rate at discharge of 75%1

*Paid testimonial by an Epicel patient. Individual results may vary.
Epicel is indicated for adult and pediatric patients who have deep dermal or full thickness burns comprising a total body surface area greater than or equal to 30%
Large Burns
≥30% TBSA
Epicel is widely reimbursed and included on a majority of national insurance plans.
Click here to learn more about Epicel reimbursement
Epicel (cultured epidermal autografts) is authorized for use in adult and pediatric patients who have deep dermal or full thickness burns comprising a total body surface area greater than or equal to 30%. It may be used in conjunction with split-thickness autografts, or alone in patients for whom split-thickness autografts may not be an option due to the severity and extent of their burns. The effectiveness of the device for this use has not been demonstrated.
Epicel is contraindicated in patients with a history of anaphylaxis following exposure to vancomycin, amikacin, and amphotericin, as trace quantities of these anti-infective agents may remain in the Epicel autograft. Epicel should not be used in patients with known sensitivities to materials of bovine or murine origin. It is contraindicated for use on clinically infected wounds.
Because Epicel is manufactured with and contains residual amounts of murine cells, FDA considers it a xenotransplantation product. Therefore, recipients should not donate whole blood, blood components, source plasma, source leukocytes, tissues, breast milk, ova, sperm, or other body parts for use in humans because there is a potential risk of carrying an infection that is transmitted from mouse cells to humans.
Squamous cell carcinoma (SCC) has been reported in patients with burn injury after being grafted with Epicel. Although SCC is a known complication of burn scars, the role of Epicel in the causation of SCC cannot be excluded.
The Epicel product is intended solely for autologous use. Patients undergoing the surgical procedure associated with Epicel are not routinely tested for transmissible infectious diseases. Discontinue use if the patient shows evidence of allergic reaction.
If clinical signs of infection are present or develop, do not apply Epicel until the infection is adequately treated.
The effectiveness of Epicel has not been proven in clinical studies.
The long-term safety of Epicel is unknown. Over the past 27 years, the mortality from all causes was 13% before hospital discharge.
Men and women who intend to have children should be advised that the effects, if any, of Epicel on fetal development have not been assessed. In addition, the safety of Epicel has not been studied in pregnant and nursing women.
Patient information supplied by attending burn teams from 1989 to 1996 reported the adverse events of highest incidence as: death (13%), infection (13.8%), graft tear (7.8%) or graft blister (4.2%) and drainage (3.3%). Some of these events may have been due to the underlying burn injury and not the device itself.
From June 1998 through September 2015 adverse events received by Genzyme Biosurgery (predecessor in interest to Vericel) and Vericel Corporation were similar to the previously identified adverse events. Events that were reported in ≥ 1% of patients included multi-organ failure (6.6%), sepsis (5.2%) infection (4%) and skin graft failure/graft complication (2.7%). The relationship of these events to Epicel has not been established.
Please see Patient Information and Instructions for Use for more information
Submission of this form constitutes your agreement to receive updates regarding Vericel products and services by email, mail, fax, telephone including pre-recorded or autodialed calls, or text (message and data rates may apply). For additional information regarding how your information may be used, and how to contact Vericel with questions or to exercise your rights, see Vericel’s Privacy Policy and Terms and Conditions of Use. You understand you may opt out of receiving information within future communications.
For additional information regarding how your information may be used, and how to contact Vericel with questions or to exercise your rights, see Vericel’s Privacy Policy and Terms and Conditions of Use. You understand you may opt out of receiving information within future communications.